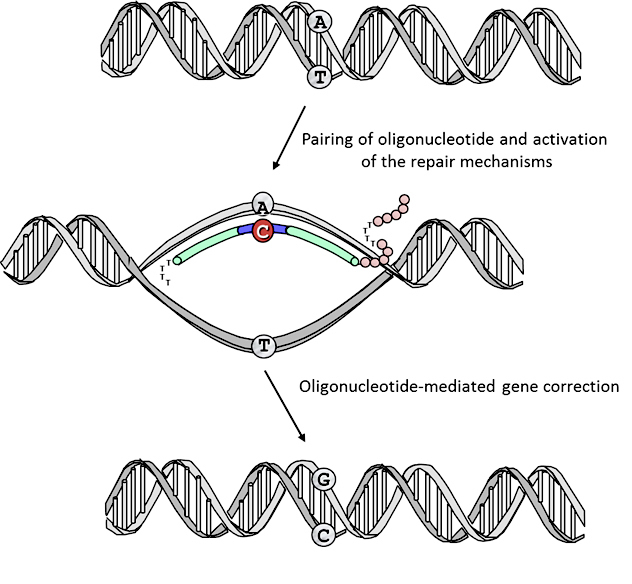
Gene Editing Strategies for DMD
Our research group has pioneered the use of gene editing strategies for the dystrophin gene to permanently correct the DNA: the source of the problem. DNA contains the information needed by every cell, including muscles, to function properly. In DMD patients the DNA that makes up the dystrophin gene contains errors. We can use oligonucleotides to let the muscle know of those errors and give the opportunity to the cell that compose each muscle to correct the mistake. We have shown that oligonucleotides can treat mouse models for DMD.
Work in the laboratory is now focused on better understanding the repair mechanism mediated by oligonucleotides and at improving the efficacy of the correction process by recruiting specific repair mechanisms that are highly active in muscle cells and that can specifically direct the repair process on the strand of the genomic DNA targeted for repair. Using state-of-the-art technologies, we are designing new tools to enhance gene repair and exploring the potential of correcting muscle stem cells as a viable approach for the treatment of the disease.
Suppression of Nonsense Mutations
Approximately 13% of all inherited genetic disorders and 15% of the DMD defects are caused by nonsense mutations that lead to prematurearrest of protein synthesis. Nonsense mutations are single point mutations in a sequence of DNA that encodes for a gene that lead to the inappropriate presence of specific sequences called stop codons (UAA, UAG or UGA). These stop codons cause a premature arrest in the synthesis of the dystrophin protein. As a result, no dystrophin is produced in skeletal muscles and heart. A major focus of our laboratory is to develop drugs that could be used to read-through premature stop codons and restore normal, full-length dystrophin protein.
We have several new drugs that are currently being tested in animal models for the disease. Our goal is to develop a drug that not only is effective on all stop codons,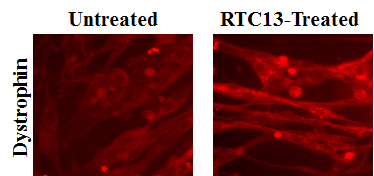 but also safe to use in patients. We have established several collaborations with expertise in medicinal chemistry, protein chemistry, medical genetics, pathology, muscle physiology and toxicology. These collaborations will guarantee the successful completion of the study in the most cost effective and sensitive manner. The ultimate goal is to enter into licensing, distribution, or partnering agreements with pharmaceutical companies that have large, established sales organizations so as to guarantee that the drug that shows to be the best candidate for the treatment of DMD reaches the clinic.
but also safe to use in patients. We have established several collaborations with expertise in medicinal chemistry, protein chemistry, medical genetics, pathology, muscle physiology and toxicology. These collaborations will guarantee the successful completion of the study in the most cost effective and sensitive manner. The ultimate goal is to enter into licensing, distribution, or partnering agreements with pharmaceutical companies that have large, established sales organizations so as to guarantee that the drug that shows to be the best candidate for the treatment of DMD reaches the clinic.
Enhancing Regeneration in Skeletal Muscles
Muscle wasting is a phenomenon common to many disorders and is particular prevalent in neurodegenerative diseases like Duchenne. The use of stem cells or muscle progenitor cells for transplantation purposes has potential for the treatment of DMD due to their high regenerative capacity and their ability of restoring muscle lost due to the disease. The results obtained in animal models for DMD and in clinical trials however, have been disappointing. The large number of cells required to achieve and effect and the inability of transplanted cells to efficiently reconstitute muscles upon transplantation hamper the development of effective treatments to DMD. The laboratory is interested in identifying genetic targets that can be used to improve muscle regeneration upon transplantation and that could significantly enhance the regenerative capacity of transplanted cells.
Using High throughput screening (HTS) technologies we have identified several candidate genes that play active role during terminal differentiation of muscle precursor cells. 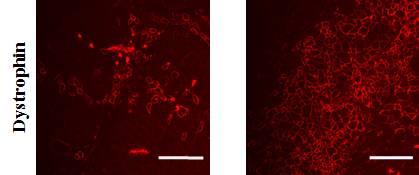 Inhibition of expression of those genes using RNA interference showed to promote myoblasts fusion in vitro and following transplantation into dystrophin deficient mice highlighting their importance for therapeutic purposes. We are now interested in determining the function of those genes and their role in myogenesis toward the development of effective therapies to DMD. These studies are likely to have a broad range of applications and are relevant to all forms of muscle disorders characterized by loss of muscle mass.
Inhibition of expression of those genes using RNA interference showed to promote myoblasts fusion in vitro and following transplantation into dystrophin deficient mice highlighting their importance for therapeutic purposes. We are now interested in determining the function of those genes and their role in myogenesis toward the development of effective therapies to DMD. These studies are likely to have a broad range of applications and are relevant to all forms of muscle disorders characterized by loss of muscle mass.